- Why Mining Matters
- Jobs
- Safety
- Environment & Operations
- FAQ
- Links
- Fun Stuff

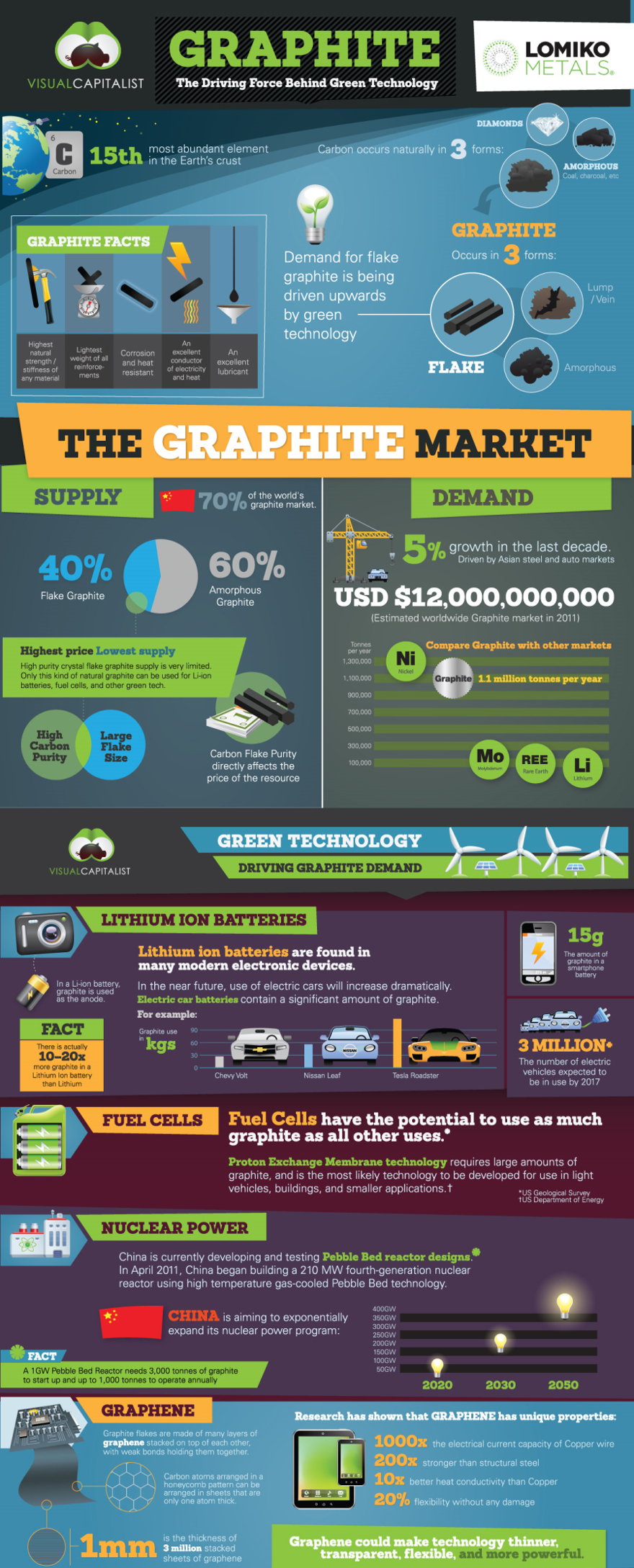
You probably don’t know it but you have used graphite to write exams and relied on it to keep your electronics powered up.
Graphite is also the reason we discovered the wonder material graphene, which is considered to be the world's thinnest, strongest and most conductive material.
Here is everything you ever wanted to know about ... GRAPHITE!
FAST FACTS!

- “Lead” pencils do not actually have lead in them. Graphite is the dark material that marks the paper in what we call lead pencils.
- Graphite is made of layers of carbon atoms. These layers can slide over each other very easily. This means that graphite is very soft.
- Graphite is a key ingredient in lithium batteries, which are essential for electric vehicles and other battery-powered devices.
- Graphite can be turned into diamond with enough heat and pressure. This is how synthetic (man-made) diamonds are made.
- Graphene, which can be derived from graphite, is the thinnest material ever devised — a pile of a million sheets of graphene would only stand as tall as the thickness of a human hair. Despite this, it is 200 times stronger than steel, and the most conductive material in the world.

Graphene is one atomic layer of graphite and it can be derived from graphite. Graphene is considered to be the world's thinnest, strongest and most conductive material - to both electricity and heat.
For example, graphene is the strongest material ever found; it is more than 40 times stronger than diamond and more than 200 times stronger than steel.
These properties give graphene the potential to revolutionize entire industries - in the fields of electricity, conductivity, energy generation, batteries, sensors and more.
Mechanical strength
Graphene is the world's strongest material, and so can be used to enhance the strength of other materials. Dozens of researchers have demonstrated that adding graphene to plastics, metals or other materials can make these materials much stronger - or lighter (as you can use less amount of material to achieve the same strength).
Such graphene-enhanced composite materials can find uses in aerospace, building materials, mobile devices, and many other applications.
Thermal applications
Graphene is the world's most conductive material to heat. As graphene is also strong and light, it means that it is a great material to make heat-spreading solutions, such as heat sinks. This could be useful in both microelectronics (for example to make LED lighting more efficient and longer lasting) and also in larger applications - for example thermal foils for mobile devices.
Energy storage
Because graphene is the world's thinnest material, it is also the material with the highest surface-area to volume ratio. This makes graphene a very promising material to be used in batteries and supercapacitors. Graphene may enable devices that can store more energy - and charge faster, too. Graphene can also be used to enhance fuel-cells.
Coatings, sensors, electronics and more
Graphene has a lot of other promising applications: anti-corrosion coatings and paints, efficient and precise sensors, faster and efficient electronics, flexible displays, efficient solar panels, faster DNA sequencing, drug delivery, and more.
Graphene is such a great and basic building block that it seems any industry can benefit from this new material. Time will tell where graphene will indeed make an impact - or whether other new materials will be more suitable.